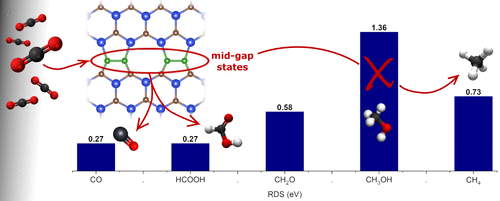
This study explores the catalytic properties of boron- and nitrogen-doped 585 extended line defects (585-ELD) in SiC monolayers for the CO2 reduction reaction (CO2RR). Using Density Functional Theory and ab initio Molecular Dynamics (AIMD) simulations, we analyze the stability, electronic structure, and adsorption characteristics of each doped defect. The results indicate that all doped systems, except for N–N, exhibit significant kinetic and thermodynamic stability, with midgap states that enhance electron availability and catalytic activity. Among the doped structures, the C–B ELD system uniquely balances CO2 protonation and H2 desorption, selectively favoring CO2 reduction over hydrogen evolution. Calculated reaction free energies show that CH4 formation is possible if the transition from H2COH to CH2 occurs, with a limiting potential (UL) of 0.73 V, while strong interactions between H2COH and the surface make CH3OH formation energetically challenging. These findings position the C–B ELD SiC system as a promising candidate for efficient and selective CO2 conversion, enabling the formation of valuable hydrocarbons and oxygenates through effective charge transfer and controlled reaction pathways.
Referência
- MORAIS, W. P.; INACIO, G. J.; ALMEIDA, E. A. R. de; SOUZA, F. A. L. de; PANSINI, F. N. N.; PAZ, W. S.
CO₂ Reduction Reactivity on the SiC Monolayer with Doped Topological Defects.
Energy & Fuels, v. 39, n. 12, 18 mar. 2025. - DOI: https://doi.org/10.1021/acs.energyfuels.4c05828.